Parasite Diagnostics Laboratory
Accurate
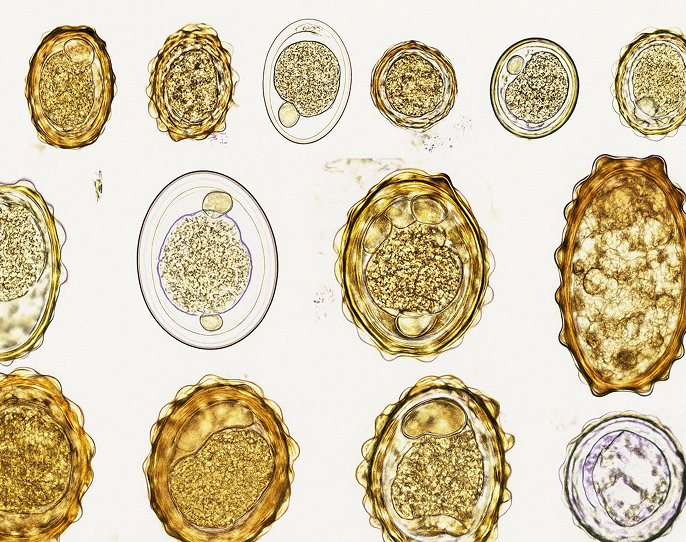
Stool Parasite Testing
for Laboratories and Clinics Worldwide
Our lab combines AI-powered microscopy and PCR for highly accurate parasite detection.
Turnaround time 3 days
AI-powered microscopy and PCR in one test
Global sample shipping available
EU-based accredited medical laboratory

.png)
.png)
.png)